Lefamulin—A New Antibiotic for Community-Acquired Pneumonia ,
Preeti N. Malani, MD, MSJ1,2
Author Affiliations Article Information
1Division of Infectious Diseases, Department of Internal Medicine, University of Michigan, Ann Arbor
2Associate Editor, JAMA
JAMA. Published online September 27, 2019. doi:10.1001/jama.2019.16215
https://www.nabriva.com/pipeline-research
Articles
Arobust antibiotic pipeline is essential for patient care and public health. Yet compared with other classes of drugs, the development of antibiotics presents unique scientific, regulatory, and economic challenges. Most notably, antibiotics provide less financial reward for pharmaceutical companies because these medications are used for a short duration and newer agents are often restricted for use only in the setting of antimicrobial resistance. In fact, most large pharmaceutical companies have reduced or stopped antibiotic research altogether, leaving the critical task of discovering new antibiotics to small companies with limited budgets and research capacity. For these and other reasons, the development and approval of a new antibiotic is a rare occurrence and a reason to celebrate.
In this issue of JAMA, Alexander et al1 report the findings of the Lefamulin Evaluation Against Pneumonia 2 (LEAP 2) trial, a phase 3, randomized, noninferiority trial that compared 5-day oral lefamulin with 7-day oral moxifloxacin in the management of community-acquired bacterial pneumonia (CABP). In this trial of 738 patients, the primary outcome of early clinical response at 96 hours (within a 24-hour window) after the first dose of study drug was 90.8% in the lefamulin group and 90.8% in the moxifloxacin group, a difference that met the noninferiority margin of 10%. Patients in the lefamulin group reported a higher incidence of gastrointestinal-related treatment-emergent adverse effects (17.9% vs 7.6% in the moxifloxacin group), primarily diarrhea.
Lefamulin (both intravenous and oral formulation) was approved in August 2019 by the US Food and Drug Administration (FDA) to treat CABP. This approval was based on data in the current LEAP 2 study as well as the previously published LEAP 1 study.2 Although lefamulin will offer another oral option to treat CABP, the spectrum of activity is similar to fluoroquinolones. In recent years, more attention has been given to uncommon but serious adverse effects associated with fluoroquinolone use including Q-T prolongation, hypoglycemia, and tendinitis and tendon rupture.
Cost will likely be a barrier to lefamulin use. A press release from the manufacturer stated that the wholesale acquisition cost of lefamulin will be $205 per day for intravenous treatment and $275 per day for oral treatment.3 This is several-fold more than moxifloxacin or levofloxacin, which are the most commonly prescribed fluoroquinolones for CABP. Tolerability (especially diarrhea and vomiting) may be another issue, and deserves close postmarketing monitoring. Despite these concerns, lefamulin is an important addition to the current antibiotic armamentarium, especially because bacterial pneumonia remains one of the most common indications for antibiotic use.4,5
Back to topArticle Information
Published Online: September 27, 2019. doi:10.1001/jama.2019.16215
Corresponding Author: Preeti N. Malani, MD, MSJ, 4135F University Hospital South, 1500 East Medical Center Dr, Ann Arbor, MI 48109 (pmalani@umich.edu).
Conflict of Interest Disclosures: None reported.
References
1.
Alexander E, Goldberg L, Das A, et al. Oral lefamulin vs moxifloxacin for early clinical response among adults with community-acquired bacterial pneumonia: the LEAP 2 randomized clinical trial [published September 27, 2019]. JAMA. doi:10.1001/jama.2019.15468
ArticleGoogle Scholar
2.
File TM, Goldberg L, Das A, et al. Efficacy and safety of intravenous-to-oral lefamulin, a pleuromutilin antibiotic, for the treatment of community-acquired bacterial pneumonia: the phase III Lefamulin Evaluation Against Pneumonia (LEAP 1) trial [published February 4, 2019]. Clin Infect Dis. doi:10.1093/cid/ciz090PubMedGoogle Scholar
3.
Nabriva. Press release. https://investors.nabriva.com/news-releases/news-release-details/nabriva-therapeutics-receives-us-fda-approval-xenleta. Accessed September 15, 2019.
4.
Magill SS, Edwards JR, Beldavs ZG, et al; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312(14):1438-1446. doi:10.1001/jama.2014.12923
ArticlePubMedGoogle ScholarCrossref
5.
Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi:10.1001/jama.2016.4151
Preeti N. Malani, MD, MSJ1,2
Author Affiliations Article Information
1Division of Infectious Diseases, Department of Internal Medicine, University of Michigan, Ann Arbor
2Associate Editor, JAMA
JAMA. Published online September 27, 2019. doi:10.1001/jama.2019.16215
https://www.nabriva.com/pipeline-research
Lefamulin
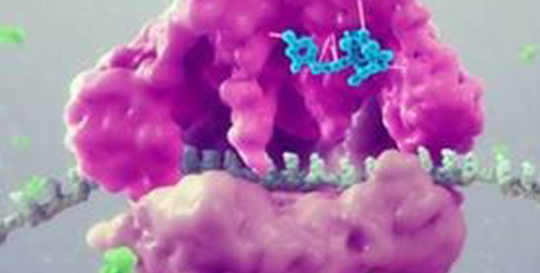
This semi-synthetic compound inhibits the synthesis of bacterial protein, which is required for bacteria to grow. It acts by binding to the peptidyl transferase center, or PTC, on the bacterial ribosome in such a way that it interferes with the interaction of protein production at two key sites known as the “A” site and the “P” site, resulting in the inhibition of bacterial proteins and the cessation of bacterial growth. Its binding occurs with high affinity, high specificity and at molecular sites that are different than other antibiotic classes.

We have completed two pivotal Phase 3 trials evaluating the safety and efficacy of lefamulin in for the treatment of adults with community-acquired bacterial pneumonia (CABP). In our first clinical trial in patients with CABP (LEAP-1), seven-days of intravenous (IV) to oral lefamulin in adults with moderate to severe CABP was compared to moxifloxacin (IV/oral), with or without linezolid. In the LEAP 2 trial, five-days of oral lefamulin was compared to seven-days of oral moxifloxacin in adults with moderate CABP. Both trials were multi-center, randomized, controlled, double-blind and enrolled patients globally. Lefamulin met all U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) primary endpoints in both LEAP 1 and LEAP 2 and was shown to be generally well tolerated. As a result of the favorable safety and tolerability profile we have observed in our clinical trials to date, we believe Lefamulin will present fewer potential complications as than current therapies. In December 2018, we completed the submission of two New Drug Applications (NDAs) to the FDA for the oral and IV formulations of lefamulin for the treatment of CABP. In february 2019, these applications were accepted by the FDA, granted priority review and given a PDUFA date of August 19, 2019. Both formulations of lefamulin were granted Qualified Infectious Disease Product (QIDP) and Fast Track designation by the FDA. In May 2019, we submitted a marketing authorization application for both IV and oral formulations of lefamulin, for the treatment of community acquired pneumonia (CAP) in adults 18 years of age and older, to the Eurpoean Medicines Agency. enabling potential Priority Review of the NDAs by the FDA. On August 19, 2019, the FDA Approved Xenleta™(lefamulin) for both Oral And IV use. Nabriva intends to work with a commercial partner to make lefamulin available to patients in the European Union.
Based on our research, we also believe that the availability of both IV and oral formulations of lefamulin, and an option to switch to oral treatment, could reduce the length of a patient’s hospital stay and the overall cost of care. Based on a combined analysis of the U.S. Centers for Disease Control and Prevention’s 2007 National Ambulatory Medical Care Survey and 2013 data from the Healthcare Cost and Utilization Project, Nabriva Therapeutics estimates that more than 5 million adults visit a site of care for CABP treatment in the United States each year. Based on 2013 data from the Healthcare Cost and Utilization Project, Nabriva Therapeutics estimates that approximately 3 million of these adult CABP patients are diagnosed in a hospital setting, where most are then treated as in-patients with IV and oral antibiotics or as Transition of Care out-patients with oral antibiotics following hospital discharge or release. To learn more about CABP and why new treatments are needed, click here.
Nabriva owns exclusive, worldwide rights to lefamulin.
We intend to further pursue the development of this antibiotic and are developing a formulation that is also appropriate for pediatric use. We believe that lefamulin's product profile also provides the opportunity to expand to additional indications beyond pneumonia, such as the treatment of acute bacterial skin and skin structure infections (ABSSSI), sexually transmitted infections (STIs), ventilator-associated bacterial pneumonia (VABP), hospital-acquired bacterial pneumonia (HABP), osteomyelitis, and prosthetic joint infections.
Lefamulin is an investigational medication that has not been found by the U.S. FDA to be safe or effective for any indication.
Articles
Arobust antibiotic pipeline is essential for patient care and public health. Yet compared with other classes of drugs, the development of antibiotics presents unique scientific, regulatory, and economic challenges. Most notably, antibiotics provide less financial reward for pharmaceutical companies because these medications are used for a short duration and newer agents are often restricted for use only in the setting of antimicrobial resistance. In fact, most large pharmaceutical companies have reduced or stopped antibiotic research altogether, leaving the critical task of discovering new antibiotics to small companies with limited budgets and research capacity. For these and other reasons, the development and approval of a new antibiotic is a rare occurrence and a reason to celebrate.
In this issue of JAMA, Alexander et al1 report the findings of the Lefamulin Evaluation Against Pneumonia 2 (LEAP 2) trial, a phase 3, randomized, noninferiority trial that compared 5-day oral lefamulin with 7-day oral moxifloxacin in the management of community-acquired bacterial pneumonia (CABP). In this trial of 738 patients, the primary outcome of early clinical response at 96 hours (within a 24-hour window) after the first dose of study drug was 90.8% in the lefamulin group and 90.8% in the moxifloxacin group, a difference that met the noninferiority margin of 10%. Patients in the lefamulin group reported a higher incidence of gastrointestinal-related treatment-emergent adverse effects (17.9% vs 7.6% in the moxifloxacin group), primarily diarrhea.
Lefamulin (both intravenous and oral formulation) was approved in August 2019 by the US Food and Drug Administration (FDA) to treat CABP. This approval was based on data in the current LEAP 2 study as well as the previously published LEAP 1 study.2 Although lefamulin will offer another oral option to treat CABP, the spectrum of activity is similar to fluoroquinolones. In recent years, more attention has been given to uncommon but serious adverse effects associated with fluoroquinolone use including Q-T prolongation, hypoglycemia, and tendinitis and tendon rupture.
Cost will likely be a barrier to lefamulin use. A press release from the manufacturer stated that the wholesale acquisition cost of lefamulin will be $205 per day for intravenous treatment and $275 per day for oral treatment.3 This is several-fold more than moxifloxacin or levofloxacin, which are the most commonly prescribed fluoroquinolones for CABP. Tolerability (especially diarrhea and vomiting) may be another issue, and deserves close postmarketing monitoring. Despite these concerns, lefamulin is an important addition to the current antibiotic armamentarium, especially because bacterial pneumonia remains one of the most common indications for antibiotic use.4,5
Back to topArticle Information
Published Online: September 27, 2019. doi:10.1001/jama.2019.16215
Corresponding Author: Preeti N. Malani, MD, MSJ, 4135F University Hospital South, 1500 East Medical Center Dr, Ann Arbor, MI 48109 (pmalani@umich.edu).
Conflict of Interest Disclosures: None reported.
References
1.
Alexander E, Goldberg L, Das A, et al. Oral lefamulin vs moxifloxacin for early clinical response among adults with community-acquired bacterial pneumonia: the LEAP 2 randomized clinical trial [published September 27, 2019]. JAMA. doi:10.1001/jama.2019.15468
ArticleGoogle Scholar
2.
File TM, Goldberg L, Das A, et al. Efficacy and safety of intravenous-to-oral lefamulin, a pleuromutilin antibiotic, for the treatment of community-acquired bacterial pneumonia: the phase III Lefamulin Evaluation Against Pneumonia (LEAP 1) trial [published February 4, 2019]. Clin Infect Dis. doi:10.1093/cid/ciz090PubMedGoogle Scholar
3.
Nabriva. Press release. https://investors.nabriva.com/news-releases/news-release-details/nabriva-therapeutics-receives-us-fda-approval-xenleta. Accessed September 15, 2019.
4.
Magill SS, Edwards JR, Beldavs ZG, et al; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312(14):1438-1446. doi:10.1001/jama.2014.12923
ArticlePubMedGoogle ScholarCrossref
5.
Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi:10.1001/jama.2016.4151

Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου