TECENTRIQ (atezolizumab) is leading the next era of treatment for locally advanced or metastatic urothelial carcinoma (mUC). Results from the prospective, international, multicentre, SAUL trial support the safety and efficacy of TECENTRIQ in a real-world mUC patient population, including patient groups that present in routine clinical practice but are usually excluded from clinical trials.1
SAUL enrolled a broad patient population with locally advanced or metastatic cancer of the bladder, renal pelvis, ureter or urethra. This included patients with:
• Predominant transitional cell histology ECOG PS 0–1 • Progression on prior platinum treatment • Creatinine clearance ≥ 30 mL/min SAUL STUDY: 46% of patients were alive at 1 year with TECENTRIQ1
For patients with disease characteristics similar to those participating in the IMvigor211* study median overall survival (OS) was 10 months (n= 643).
|
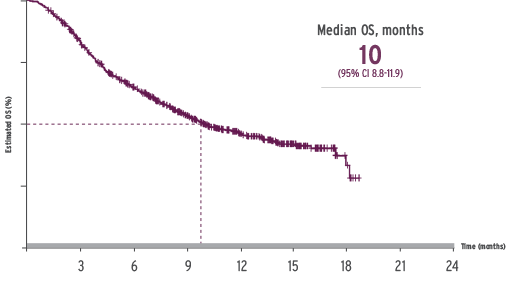 |
*Defined as the SAUL intent-to-treat population minus the populations excluded from IMvigor211. IMvigor211 was an open-label, multicentre, international, randomised trial that assessed the efficacy and safety of TECENTRIQ in locally advanced or metastatic urothelial carcinoma patients who progressed during or following a platinum-containing regimen.
SAUL
• Disease control rate was 40% in previously-treated mUC patients • Median duration of response was not reached after 12 months follow up The expert’s view
Prof. Axel Merseburger discusses the recently published SAUL data presented at the European Association of Urology 2019 Congress held in Barcelona, Spain.
|
 |
TECENTRIQ: well tolerated in a complex UC population1
The SAUL trial established TECENTRIQ’s safety profile across a wide mUC population with a range of comorbidities.
Most common treatment-emergent AEs (occurring in >10% of patients) by grade.
|
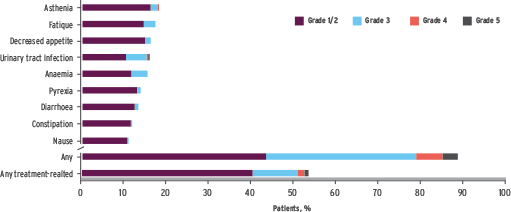 |
Median no. of cycles: 5 (range 1-28). Median treatment duration: 2.8 months (range 0-19 months)
Treatment-related grade 5 AEs (n=7, 0.7%): two cases of dyspnoea, one case each of colitis, intestinal perforation, respiratory failure, chronic kidney disease, drug-induced liver injury. Most common treatment-related grade ≥3 AEs: fatigue, asthenia, colitis, hypertension (each 1%)
• Low incidence of grade 3 treatment-related adverse events
• <1% of patients discontinued treatment due to an immune-related adverse event |

Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου